What property did Moseley use to organize his periodic table?
Here is a SUPER like shooting fish in a barrel Caption + Comparison on Mendeleev and Moseley Periodic table.
In fact, I will also show you the old age original picture of Periodic systems which was published by Mendeleev in the yr 1869.
Then permit'southward finish this very quickly.
Mendeleev's Periodic System

I know you can't read annihilation from this flick, but this is the original arrangement of elements which was done by Dmitri Mendeleev in 1869.
Allow me evidence you a articulate picture.
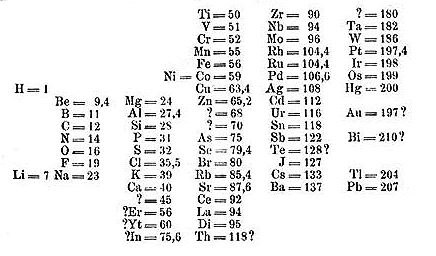
In Mendeleev'south Periodic table, the elements were arranged in the increasing lodge of their atomic masses.
Only 63 elements were discovered during Mendeleev's time, so he made the arrangement of those 63 elements in his tabular array (which is shown higher up).
Moseley'southward Periodic table
Moseley's Periodic table was developed past Henry Gwyn Jeffreys Moseley in the year 1914.
During his fourth dimension, the atomic construction was known, so he had a clear idea about the protons, neutrons and electrons.
He institute that the protons are the unique identity for each and every chemical element, and the number of protons (or atomic number) decides the chemical properties of elements.
And hence he arranged the known elements in the increasing order of their atomic number.
Moseley'southward Periodic table gave usa the clear concept for the system of elements.
And because of his valuable efforts, today we take a complete periodic tabular array with 118 elements without any limitations in arrangement.
Difference between Mendeleev and Moseley Periodic table
| Mendeleev's Periodic table | Moseley'south Periodic table |
|---|---|
| i). Organisation of elements is based on atomic mass . | 1). Arrangement of elements is based on atomic number . |
| Caption of 1st point The elements in the Mendeleev's Periodic table were arranged in the increasing social club of their atomic mass. While in the Moseley's Periodic table, the elements were arranged in the increasing order of their diminutive number. | |
| 2). Published in the year 1869. | 2). Published in the twelvemonth 1914. |
| Explanation of 2nd point Mendeleev's Periodic table and his observation on the Periodic arrangement was presented to the Russian chemical lodge in the year 1869. While Moseley'due south Periodic table was published afterward in the yr 1914. | |
| 3). Only 63 elements were nowadays in Mendeleev's Periodic table in 1869. | 3). Effectually 92 elements were present in Moseley's Periodic table in 1914. |
| Explanation of 3rd betoken At that place were only 63 elements discovered during the time of Mendeleev (1869). And so his Periodic tabular array contains simply 63 elements. During the time of Moseley (1914), there were around 92 elements discovered. So the Periodic table presented by Moseley had 92 elements. | |
| iv). It has 8 groups & 6 periods. | 4). Information technology has 18 groups & seven periods. |
| Caption of 4th bespeak Really the originally published Mendeleev'southward Periodic table had no rows and columns (come across below image).  Just later on, for improve understanding, the modified version was presented which contains 8 columns (groups) and half-dozen rows (periods). 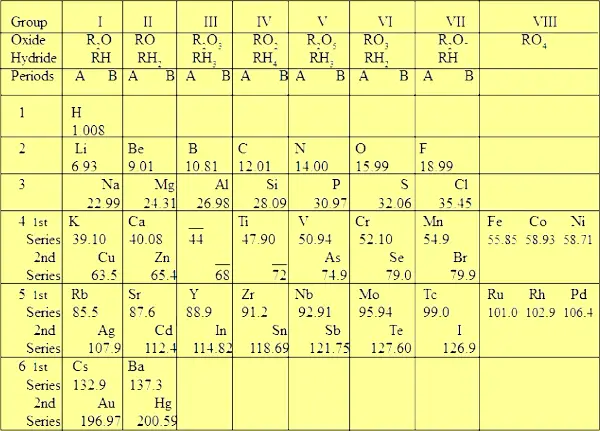 Out of the 6 periods, the quaternary, 5th and sixth periods (rows) are over again subdivided into 2 series named "first series" and "second series". That'due south why y'all tin run into two elements in a unmarried block (come across above image). While in the Moseley'south Periodic table, in that location are 18 groups and seven periods. There is nothing like subdivisions in Moseley's Periodic tabular array. | |
| v). Mendeleev left three gaps for the undiscovered elements. | 5). There were 7 gaps in Moseley's Periodic table. |
| Explanation of fifth bespeak In 1869, when Mendeleev published a Periodic tabular array, there were merely 63 elements discovered at that time. While arranging these known elements, he left 3 gaps for those elements whose backdrop were not matching with the previous ones. And so he didn't place those elements in the same column. Hence, he left 3 gaps in his Periodic table. He named those elements every bit eka-boron, eka-aluminium and eka-silicon. Later on, these elements were discovered and today nosotros recognize those elements as scandium, gallium and germanium respectively. Similarly during the time of Moseley, in 1914, in that location were few undiscovered elements too. | |
| 6). Nomenclature was based on hydrides and oxides formed by the chemical reaction. | vi). Classification was based on electron configuration. |
| Explanation of 6th indicate The classification of elements in the Mendeleev'due south periodic table was washed on the ground of compounds like hydrides and oxides which were formed by the chemic reaction. If the elements were having similar hydrides and oxides, they were placed in the same group by Mendeleev. While in Moseley'south Periodic tabular array, the nomenclature was done on the basis of electron configuration of the elements. | |
| 7). Mendeleev did not explicate why elements of aforementioned groups have similar chemical properties, and elements of same periods accept different properties. | vii). Moseley explained the reason behind the similar backdrop of elements in groups, and the reason was electron configuration. |
| Explanation of seventh indicate Concept: Valence electrons are the number of electrons present in the outermost orbit of an atom. Mendeleev's Periodic table did not draw the reason why elements of the aforementioned grouping have similar chemical properties and why elements of the aforementioned period have different chemic properties. While, during Moseley's time, the diminutive construction was discovered and the electronic configuration of elements were known to the chemists. | |
| eight). Noble gases are not included in Mendeleev's periodic table. | 8). Noble gases are included in the Moseley Periodic table (in group eighteen). |
| Caption of eighth point The Noble gases are the inert gases. They do not react with whatsoever other elements. Hence it is very difficult to find Noble gases in the compound form. Besides the quantity of Noble gases on the world is very very less. Then Noble gases were not discovered during Mendeleev's fourth dimension. And equally they were non discovered, nosotros do not discover any Noble gases in Mendeleev's tabular array. While during Moseley'due south fourth dimension, the Noble gases were discovered. Hence he included them in separate group 18 on his table. | |
| 9). No positions for isotopes in Mendeleev's tabular array. | ix). The isotopes have same position along with the neutral atom. |
| Caption of 9th point Isotopes are those variants of chemical elements which have the aforementioned number of protons and electrons, but different number of neutrons. Considering of this reason, isotopes accept different atomic mass as compared to the neutral atom. Mendeleev did non mention any positions for the isotopes. While in Moseley's Periodic tabular array, the isotopes were given the aforementioned position as that of the neutral cantlet. | |
| 10). Some similar elements were placed in different groups while some dissimilar elements were placed in same group. | 10). There are no such misplaced elements in Moseley's Periodic table. |
| Caption of 10th point In Mendeleev'southward periodic table, to maintain the increasing order of atomic mass, some elements which possess like chemical properties were placed in dissimilar groups, while some dissimilar elements were placed in the same group. While in Moseley'southward Periodic table, there are no such misplaced elements. | |
| 11). The properties of elements were not repeating afterward regular intervals. | 11). The backdrop of elements were repeating later regular intervals. |
| Explanation of 11th signal In Mendeleev's periodic table, the elements are bundled on the basis of atomic mass. And the fact is that the chemical properties of elements do non depend on atomic mass, but it depends on atomic number. So Mendeleev's periodic tabular array organisation do non testify repetitive properties in elements. While in Moseley'south Periodic table, the elements are arranged on the basis of atomic number, and diminutive number (actually valence electrons) only decides the chemical properties. Hence Moseley's Periodic table shows repetitive properties after regular intervals of 2, viii, 8, 18, 18,… | |
| 12). Separate positions for metals and Nonmetals were not nowadays. | 12). Metals, Nonmetals and semimetals accept dissever positions. |
| Explanation of 12th betoken In Mendeleev's periodic table, there are no separate positions for metals and nonmetals. But they are all found together. While in Moseley's Periodic table, they are at specific positions. The metals are on the left, nonmetals are at the right and semimetals are located in between them. | |
| 13). Some elements with higher atomic mass were placed earlier the elements with lower diminutive mass. | 13). All the elements were arranged in the increasing order of their diminutive number. |
| Explanation of 13th point Mendeleev made the arrangement of elements on the footing of increasing atomic masses. But to maintain the similarities in the backdrop, some elements with college atomic mass were placed before the elements with lower atomic mass. While in Moseley's Periodic table, all the elements were arranged in increasing order of atomic number. | |
| xiv). Group number and period number of elements cannot be predicted from Mendeleev'southward table. | fourteen). Grouping number and menses number of any chemical element tin can be perfectly found from Moseley'south Periodic table |
| Explanation of 14th point Mendeleev's tabular array is based on diminutive mass, but Moseley's table is based on atomic number. From atomic number, we can easily detect the electron configuration and finally we can notice the position of elements in periodic table. | |
| 15). Transition elements were constitute along with the other elements in Mendeleev's Periodic tabular array. | 15). Transition elements were classified in split up block (d block) in Moseley's Periodic table. |
| Explanation of 15th point In Mendeleev's periodic table, in that location are no block wise classification because in that location was no knowledge of electronic configuration and orbitals. Hence, there is no cake wise nomenclature of transition elements on Mendeleev's periodic table. While in Moseley'southward Periodic tabular array, the noesis of orbitals was much developed and hence we tin see southward p d and f block on the Moseley'southward Periodic tabular array. | |
Summary
I hope you have conspicuously understood the Mendeleev and Moseley Periodic tabular array.
You lot tin can besides remember the difference between Mendeleev and Moseley Periodic table from this unmarried image given below.
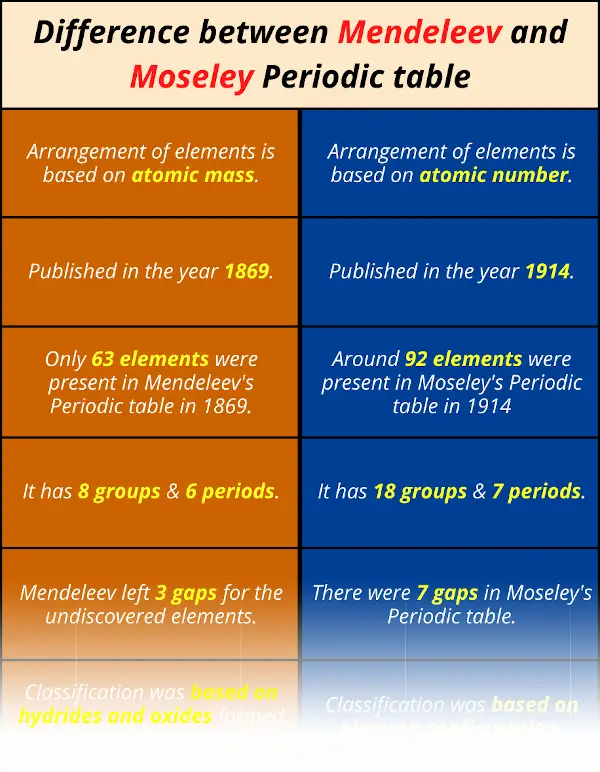
If I volition requite you all the differences in this unmarried epitome, then this image will be very long.
Hence, refer the above tabular array for all the differences (along with their explanations.)
Complimentary Souvenir for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Tabular array will help you in your studies.
1). You can effortlessly discover every single detail near the elements from this single Interactive Periodic table.
2). You volition get the detailed information about the periodic table which volition convert a newbie into pro.
3). You volition besides get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download information technology's high resolution image at present (It's FREE)
Source: https://periodictableguide.com/mendeleev-and-moseley-periodic-table/
Post a Comment for "What property did Moseley use to organize his periodic table?"